Because the oral formulation must be taken on an empty stomach, physicians may weigh convenience against efficacy when choosing between products. Novo Nordisk has stated that the restriction—patients should wait roughly 30 minutes before eating—should not pose a major obstacle to adherence.
Competition with Eli Lilly intensifies
Eli Lilly has filed its own oral GLP-1 candidate, orforglipron, with the FDA and expects a decision in March or April 2026. In a late-stage trial, Lilly’s pill produced average weight loss of 12.4% over about one year. Novo Nordisk’s semaglutide capsule demonstrated a 16.6% reduction in body weight across a comparable time frame.
Both companies are already vying for dominance in injectables. Novo Nordisk’s Ozempic, approved for type 2 diabetes in 2017, held a multiyear head start against Lilly’s Mounjaro. Despite that advantage, Lilly recently surpassed Novo Nordisk in U.S. prescription volumes as supply constraints limited availability of Ozempic and Wegovy, opening the door for compounding pharmacies and alternative providers.
Analysts note that the first-to-market position in oral treatments does not guarantee long-term leadership. Lilly’s maintenance study showing a seamless transition for patients moving from its injectable Zepbound or Wegovy to orforglipron could help the Indianapolis-based group retain share once both tablets are available.
Relief after a difficult 2025
The FDA decision arrives after a tumultuous 12 months for Novo Nordisk. The company issued multiple profit warnings, announced workforce reductions, underwent changes in senior management, and engaged in a high-profile—and ultimately unsuccessful—bidding contest with Pfizer. These setbacks contributed to a roughly 50% decline in Novo Nordisk’s share price in 2025, the steepest annual fall since the firm listed.
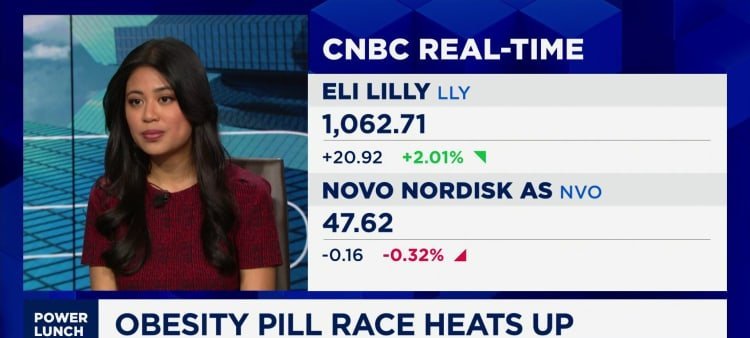
Imagem: Internet
Market observers characterized the FDA clearance as a turning point. Danish lender Jyske Bank called the outcome a “welcome Christmas gift” for shareholders and for patients who often initiate weight-loss programs at the start of a new year. HSBC analysts highlighted that the safety and tolerability profile observed in trials could give the semaglutide pill an edge, though they cautioned that execution on supply and commercialization will be decisive.
Indication spans weight loss and cardiovascular risk
The FDA label covers both long-term weight management and reduction of major adverse cardiovascular events, including heart attack and stroke, for adults who meet the eligibility criteria. The broader indication mirrors that of the injectable version, underscoring the growing recognition of GLP-1 drugs as tools not only for weight reduction but also for improving cardiac outcomes.
A surge of industry activity underscores the segment’s commercial potential. Pfizer, AstraZeneca, and Roche are among the large pharmaceutical groups pursuing their own oral or injectable obesity therapies. Research firm McKinsey estimates the global market for anti-obesity medications could exceed $100 billion annually within a decade.
Next steps
With U.S. launch preparations under way, Novo Nordisk must scale manufacturing to avoid the supply shortages that have constrained its injectable products. The company has not disclosed production capacity figures but said it is investing in additional facilities to meet anticipated demand.
Investors will monitor early prescription trends once the pill becomes available, the speed of international approvals, and the progress of Lilly’s orforglipron review. Pipeline developments from competing drugmakers also remain in focus as the scramble for share in the obesity market accelerates.
Crédito da imagem: CNBC



